Avzivi® (Bevacizumab-tnjn) , sold under the brand name POBEVCY (普贝希) in China, is a humanized monoclonal antibody that targets VEGF. It specifically binds to VEGF and blocks the binding of VEGF to its receptor, thereby reducing neovascularization, inducing the degradation of existing blood vessels, and thereby inhibiting tumor growth.
Chinese Version
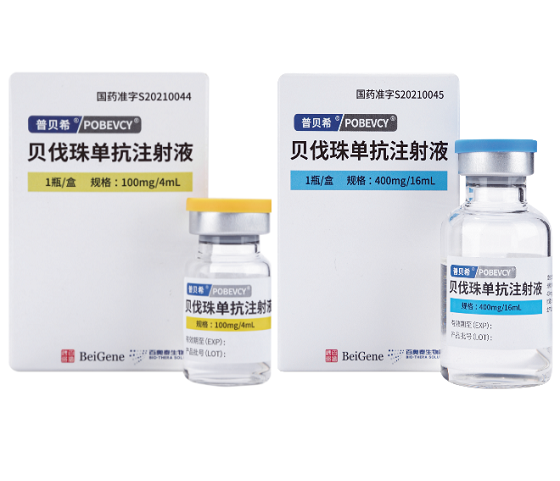
Brand name:POBEVCY (普贝希)
INN name:Bevacizumab (贝伐珠单抗注射液)
Manufacturer:Bio-Thera (百奥泰生物)
Packaging:100mg/4ml/Box
Packaging:400mg/16ml/Box
Inquire&E-mail: care@100pei.com
Dec 7, 2023,The FDA has approved the fifth bevacizumab biosimilar, Bio-Thera Solutions’ Avzivi (bevacizumab-tnjn) for the treatment of several types of cancer. Avzivi® (BAT1706) is Bio-Thera Solutions’ second FDA approved product in the United States. Avzivi® is the second biosimilar researched, developed, and manufactured by a Chinese pharmaceutical company to receive FDA approval in the United States.
 扫一扫
扫一扫